 |
Science Frontiers ONLINE No. 71: Sep-Oct 1990 |
|
|
Novel Forms Of Matter
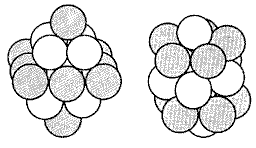 Clusters. What is the difference between "vanilla" and "chocolate" niobium? To begin with, these two forms of niobium are neither single atoms nor crystalline arrays of atoms. Both "flavors" of niobium consist of 19 niobium atoms (Nb19+ ), but the atoms are clustered differently, as illustrated. The different clusters react quite differently chemically. The "chocolate" niobium, on the right, is a capped icosahedron and reacts readily with hydrogen. The "vanilla" double pyramid (left) has flatter surfaces and does not readily combine with hydrogen.
Clusters. What is the difference between "vanilla" and "chocolate" niobium? To begin with, these two forms of niobium are neither single atoms nor crystalline arrays of atoms. Both "flavors" of niobium consist of 19 niobium atoms (Nb19+ ), but the atoms are clustered differently, as illustrated. The different clusters react quite differently chemically. The "chocolate" niobium, on the right, is a capped icosahedron and reacts readily with hydrogen. The "vanilla" double pyramid (left) has flatter surfaces and does not readily combine with hydrogen.
Cluster research is embryonic, with new surprises popping up almost every day. Once a cluster size exceeds a few hundred atoms, its properties begin to resemble those of the bulk material. However, "cluster-assembled materials" have been made by attaching clusterto-cluster in a sort of patchwork quilt. Such materials have unique properties that are quite different from those of the normal crystalline and amorphous materials. (Pool, Robert; "Clusters: Strange Morsels of Matter," Science, 248:1186, 1990.)
Comment. One would expect that the effects of clustering would be important in biology, too. We obviously have a lot to learn about this new realm between single atoms/molecules and bulk materials.
Quasicrystals. Quasicrystals, once considered physically impossible, have been found easy to grow -- once one's mindset is corrected. At Bell Labs, for example, quasicrystals of an aluminum-cobaltcopper alloy reveal atoms packed together in pentagonal arrays -- the very geometry that crystallographers assured us could not exist. As one of the Bell researchers remarked, "Most of the ap plications are unimagined." This goes for the basic properties of quasicrystals, too. (Keller, John J.; "Bell Labs Confirms That New Form of Matter Exists," Wall Street Journal, February 8, 1990. Cr. J. Covey.)
Nitrogen molecules that shouldn't exist.
"Chemists in West Germany have discovered a compound of nitrogen which breaks one of the fundamental rules of chemistry. The molecule has five bonds and is 'an extremely stable species.' According to the text books, a nitrogen atom cannot form more than four bonds."
The unruly nitrogen atom lurks at the center of a tribonal bipyramid of 5 gold atoms. Should you ask to synthesize a few of these molecular anomalies, take some (phosphine-gold) ammonium tetrafluoroborate from your shelf and add (triphenylphosphine) gold tetrafluoroborate! (Emsley, John; "The Nitrogen Molecule that Shouldn't Exist," New Scientist, p. 30, May 26, 1990.)